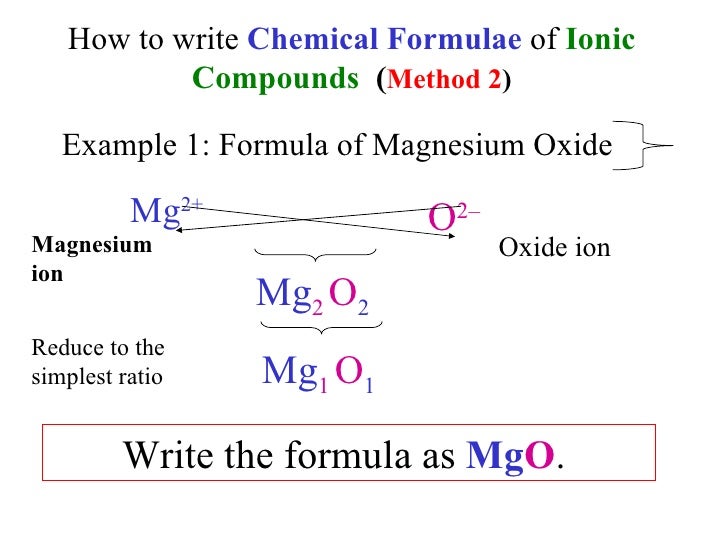
Magnesium Oxide Balanced Equation . for example, magnesium and oxygen in the air react to produce magnesium oxide. The burning of magnesium metal reacts with oxygen found. Deduce the balanced equation for the reaction. the reaction between magnesium and oxygen. magnesium reacts with oxygen to produce magnesium oxide. Magnesium + oxygen → magnesium oxide. Magnesium is in group 2 of the periodic table. Write the balanced chemical equation for this reaction. A magnesium atom will lose 2 electrons to form a stable 2 + ion. in this video we'll balance the equation mg + o2 = mgo and provide the correct. use the following information to calculate the mass of oxygen that reacted with the magnesium to form magnesium oxide. Mg(s) + o₂(g) → mgo(s) 6.08 g of magnesium reacts with 4.00 g oxygen to produce magnesium oxide, mgo. The word and symbol equations are:
from www.slideshare.net
the reaction between magnesium and oxygen. Deduce the balanced equation for the reaction. use the following information to calculate the mass of oxygen that reacted with the magnesium to form magnesium oxide. Magnesium is in group 2 of the periodic table. The burning of magnesium metal reacts with oxygen found. magnesium reacts with oxygen to produce magnesium oxide. 6.08 g of magnesium reacts with 4.00 g oxygen to produce magnesium oxide, mgo. in this video we'll balance the equation mg + o2 = mgo and provide the correct. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Magnesium + oxygen → magnesium oxide.
Chem matters ch6_ionic_bond
Magnesium Oxide Balanced Equation The word and symbol equations are: the reaction between magnesium and oxygen. in this video we'll balance the equation mg + o2 = mgo and provide the correct. The word and symbol equations are: Mg(s) + o₂(g) → mgo(s) Deduce the balanced equation for the reaction. Magnesium is in group 2 of the periodic table. for example, magnesium and oxygen in the air react to produce magnesium oxide. magnesium reacts with oxygen to produce magnesium oxide. Write the balanced chemical equation for this reaction. A magnesium atom will lose 2 electrons to form a stable 2 + ion. 6.08 g of magnesium reacts with 4.00 g oxygen to produce magnesium oxide, mgo. Magnesium + oxygen → magnesium oxide. use the following information to calculate the mass of oxygen that reacted with the magnesium to form magnesium oxide. The burning of magnesium metal reacts with oxygen found.
From www.tessshebaylo.com
Balanced Chemical Equation For Synthesis Of Magnesium Oxide Tessshebaylo Magnesium Oxide Balanced Equation 6.08 g of magnesium reacts with 4.00 g oxygen to produce magnesium oxide, mgo. Magnesium is in group 2 of the periodic table. Write the balanced chemical equation for this reaction. A magnesium atom will lose 2 electrons to form a stable 2 + ion. The word and symbol equations are: The burning of magnesium metal reacts with oxygen. Magnesium Oxide Balanced Equation.
From www.chegg.com
Solved 1. Write a balanced equation for each reaction Magnesium Oxide Balanced Equation magnesium reacts with oxygen to produce magnesium oxide. 6.08 g of magnesium reacts with 4.00 g oxygen to produce magnesium oxide, mgo. The word and symbol equations are: Mg(s) + o₂(g) → mgo(s) use the following information to calculate the mass of oxygen that reacted with the magnesium to form magnesium oxide. A magnesium atom will lose. Magnesium Oxide Balanced Equation.
From www.numerade.com
SOLVEDWrite the balanced equation for the of magnesium Magnesium Oxide Balanced Equation in this video we'll balance the equation mg + o2 = mgo and provide the correct. Mg(s) + o₂(g) → mgo(s) Deduce the balanced equation for the reaction. for example, magnesium and oxygen in the air react to produce magnesium oxide. Write the balanced chemical equation for this reaction. 6.08 g of magnesium reacts with 4.00 g. Magnesium Oxide Balanced Equation.
From www.slideshare.net
Chemical equations Magnesium Oxide Balanced Equation the reaction between magnesium and oxygen. use the following information to calculate the mass of oxygen that reacted with the magnesium to form magnesium oxide. Mg(s) + o₂(g) → mgo(s) The word and symbol equations are: in this video we'll balance the equation mg + o2 = mgo and provide the correct. Deduce the balanced equation for. Magnesium Oxide Balanced Equation.
From www.numerade.com
SOLVED Consider the balanced reaction of magnesium and oxygen 2 Mg Magnesium Oxide Balanced Equation Magnesium is in group 2 of the periodic table. for example, magnesium and oxygen in the air react to produce magnesium oxide. A magnesium atom will lose 2 electrons to form a stable 2 + ion. 6.08 g of magnesium reacts with 4.00 g oxygen to produce magnesium oxide, mgo. use the following information to calculate the. Magnesium Oxide Balanced Equation.
From www.markedbyteachers.com
The Formula for Magnesium Oxide. GCSE Science Marked by Magnesium Oxide Balanced Equation magnesium reacts with oxygen to produce magnesium oxide. Write the balanced chemical equation for this reaction. in this video we'll balance the equation mg + o2 = mgo and provide the correct. Deduce the balanced equation for the reaction. use the following information to calculate the mass of oxygen that reacted with the magnesium to form magnesium. Magnesium Oxide Balanced Equation.
From cextfphn.blob.core.windows.net
Magnesium And Oxygen Reaction at Mary Gomez blog Magnesium Oxide Balanced Equation in this video we'll balance the equation mg + o2 = mgo and provide the correct. Deduce the balanced equation for the reaction. The burning of magnesium metal reacts with oxygen found. the reaction between magnesium and oxygen. Magnesium is in group 2 of the periodic table. Write the balanced chemical equation for this reaction. The word and. Magnesium Oxide Balanced Equation.
From www.slideserve.com
PPT Chapter 8 Review Topics PowerPoint Presentation, free download Magnesium Oxide Balanced Equation magnesium reacts with oxygen to produce magnesium oxide. in this video we'll balance the equation mg + o2 = mgo and provide the correct. Deduce the balanced equation for the reaction. Magnesium + oxygen → magnesium oxide. The word and symbol equations are: Write the balanced chemical equation for this reaction. A magnesium atom will lose 2 electrons. Magnesium Oxide Balanced Equation.
From www.numerade.com
SOLVED Solid magnesium hydroxide into gaseous water and Magnesium Oxide Balanced Equation Magnesium is in group 2 of the periodic table. The word and symbol equations are: magnesium reacts with oxygen to produce magnesium oxide. for example, magnesium and oxygen in the air react to produce magnesium oxide. Mg(s) + o₂(g) → mgo(s) Magnesium + oxygen → magnesium oxide. Deduce the balanced equation for the reaction. A magnesium atom will. Magnesium Oxide Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for reaction of the basic oxide Magnesium Oxide Balanced Equation Deduce the balanced equation for the reaction. the reaction between magnesium and oxygen. Magnesium is in group 2 of the periodic table. Mg(s) + o₂(g) → mgo(s) use the following information to calculate the mass of oxygen that reacted with the magnesium to form magnesium oxide. A magnesium atom will lose 2 electrons to form a stable 2. Magnesium Oxide Balanced Equation.
From www.youtube.com
Balancing Mg + O2 → MgO YouTube Magnesium Oxide Balanced Equation in this video we'll balance the equation mg + o2 = mgo and provide the correct. Magnesium is in group 2 of the periodic table. Deduce the balanced equation for the reaction. The burning of magnesium metal reacts with oxygen found. 6.08 g of magnesium reacts with 4.00 g oxygen to produce magnesium oxide, mgo. Magnesium + oxygen. Magnesium Oxide Balanced Equation.
From www.scribd.com
When Oxygen Gas Reacts With Magnesium, Magnesium Oxide Is Formed Magnesium Oxide Balanced Equation use the following information to calculate the mass of oxygen that reacted with the magnesium to form magnesium oxide. 6.08 g of magnesium reacts with 4.00 g oxygen to produce magnesium oxide, mgo. for example, magnesium and oxygen in the air react to produce magnesium oxide. the reaction between magnesium and oxygen. Mg(s) + o₂(g) →. Magnesium Oxide Balanced Equation.
From armsingle10.pythonanywhere.com
Fabulous Magnesium Oxide Word Equation Ncea Level 3 Physics Resource Sheet Magnesium Oxide Balanced Equation use the following information to calculate the mass of oxygen that reacted with the magnesium to form magnesium oxide. the reaction between magnesium and oxygen. Write the balanced chemical equation for this reaction. The burning of magnesium metal reacts with oxygen found. Mg(s) + o₂(g) → mgo(s) Deduce the balanced equation for the reaction. Magnesium is in group. Magnesium Oxide Balanced Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For Synthesis Of Magnesium Oxide Tessshebaylo Magnesium Oxide Balanced Equation The word and symbol equations are: for example, magnesium and oxygen in the air react to produce magnesium oxide. Magnesium is in group 2 of the periodic table. A magnesium atom will lose 2 electrons to form a stable 2 + ion. 6.08 g of magnesium reacts with 4.00 g oxygen to produce magnesium oxide, mgo. in. Magnesium Oxide Balanced Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For Synthesis Of Magnesium Oxide Tessshebaylo Magnesium Oxide Balanced Equation The word and symbol equations are: Mg(s) + o₂(g) → mgo(s) Magnesium is in group 2 of the periodic table. the reaction between magnesium and oxygen. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Magnesium + oxygen → magnesium oxide. in this video we'll balance the equation mg + o2 = mgo. Magnesium Oxide Balanced Equation.
From www.youtube.com
How to Balance MgO + H2O = Mg(OH)2 (Magnesium Oxide plus Water) YouTube Magnesium Oxide Balanced Equation A magnesium atom will lose 2 electrons to form a stable 2 + ion. magnesium reacts with oxygen to produce magnesium oxide. for example, magnesium and oxygen in the air react to produce magnesium oxide. in this video we'll balance the equation mg + o2 = mgo and provide the correct. The word and symbol equations are:. Magnesium Oxide Balanced Equation.
From kelasmenggambarbagus920.blogspot.com
Magnesium Oxide + Hydrochloric Acid Balanced Equation Now Magnesium Oxide Balanced Equation Deduce the balanced equation for the reaction. Write the balanced chemical equation for this reaction. for example, magnesium and oxygen in the air react to produce magnesium oxide. A magnesium atom will lose 2 electrons to form a stable 2 + ion. magnesium reacts with oxygen to produce magnesium oxide. the reaction between magnesium and oxygen. . Magnesium Oxide Balanced Equation.
From testbook.com
Magnesium Oxide Formula Check Structure, Preparation & Uses Magnesium Oxide Balanced Equation Write the balanced chemical equation for this reaction. The word and symbol equations are: Mg(s) + o₂(g) → mgo(s) for example, magnesium and oxygen in the air react to produce magnesium oxide. Magnesium is in group 2 of the periodic table. A magnesium atom will lose 2 electrons to form a stable 2 + ion. The burning of magnesium. Magnesium Oxide Balanced Equation.